Introducing The AI Project Planner — Scope, Build, Map, Compare, and Enrich your projects with precision. Learn More →
Turn strategy into execution with Planner, the fastest way to shape life science projects.
Planner is your thought partner across the product lifecycle, from discovery to delivery.
Prompt:
Define strategic framework for ANGPTL3 base editing cardiovascular gene therapy advancing toward IND submission; establish competitive positioning against Verve's VERV-201 and CRISPR Therapeutics' CTX310; map regulatory pathway leveraging FDA's January 2024 gene therapy guidance; address ABE8e-NG platform validation, manufacturing partnerships, and safety characterization for severe refractory dyslipidemia populations.

Prompt:
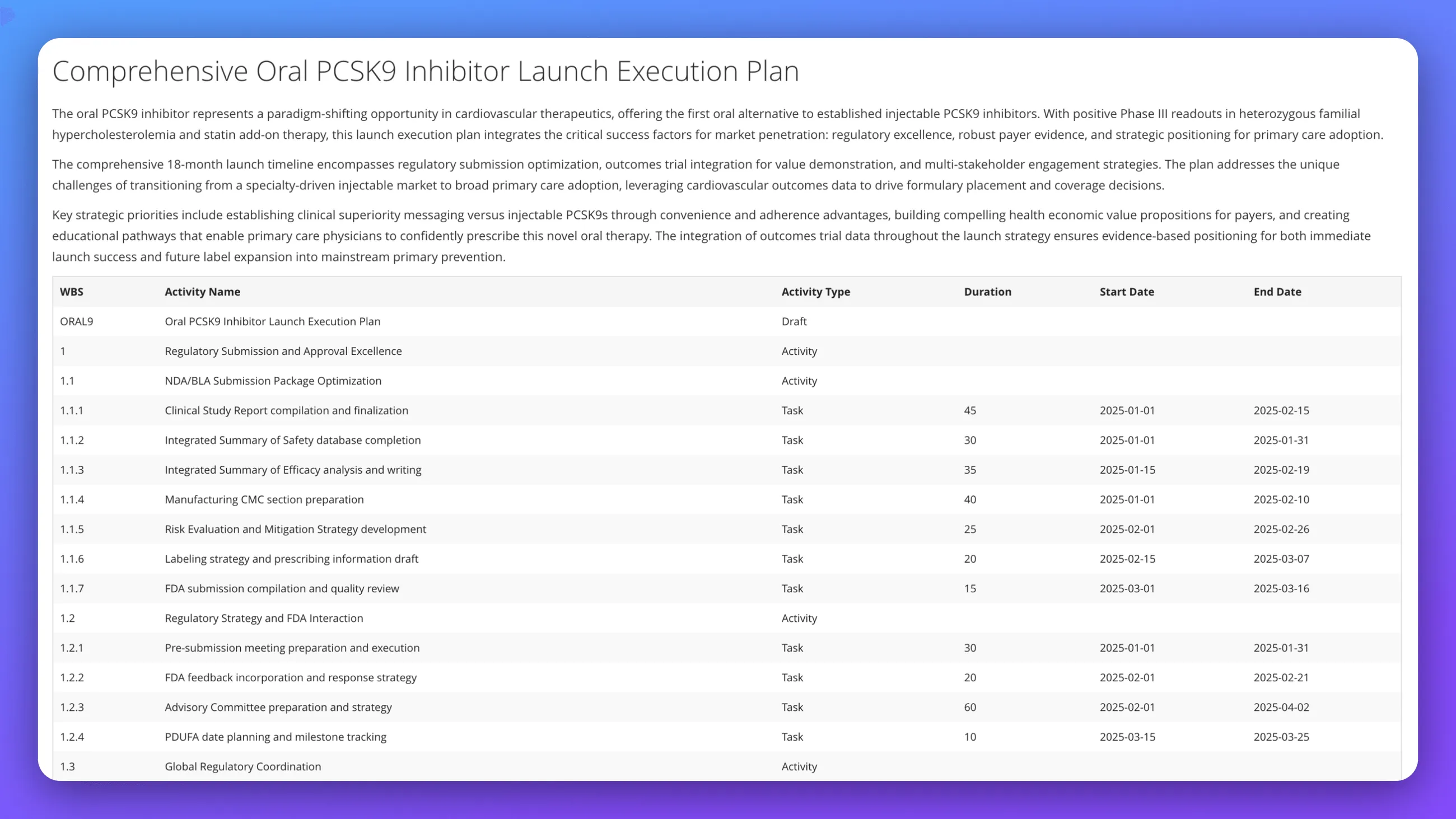
Build comprehensive launch execution plan for oral PCSK9 inhibitor advancing from Phase III readouts to commercial launch; define regulatory submission strategy with NDA/BLA package optimization, FDA interaction timeline, and global coordination; design market access approach integrating health economics, payer evidence generation, and primary care physician education; address competitive positioning against injectable PCSK9 inhibitors and launch readiness across regulatory approval, manufacturing scale-up, and commercial operations.
Prompt:
Map three strategic scenarios for pulsed field ablation system launch execution to determine optimal competitive response: Integrated Platform Acceleration Response leveraging mapping system partnerships and bundle strategies, Registry-Driven Evidence Dominance focusing on real-world evidence generation and clinical superiority, Selective Market Penetration targeting specific EP lab segments; quantify each for market share trajectory, competitive positioning against Boston Scientific and Medtronic, investment requirements, and revenue potential through 2030.

With Planner, you're in control—structure complexity when needed, or keep things simple.
Prompt:
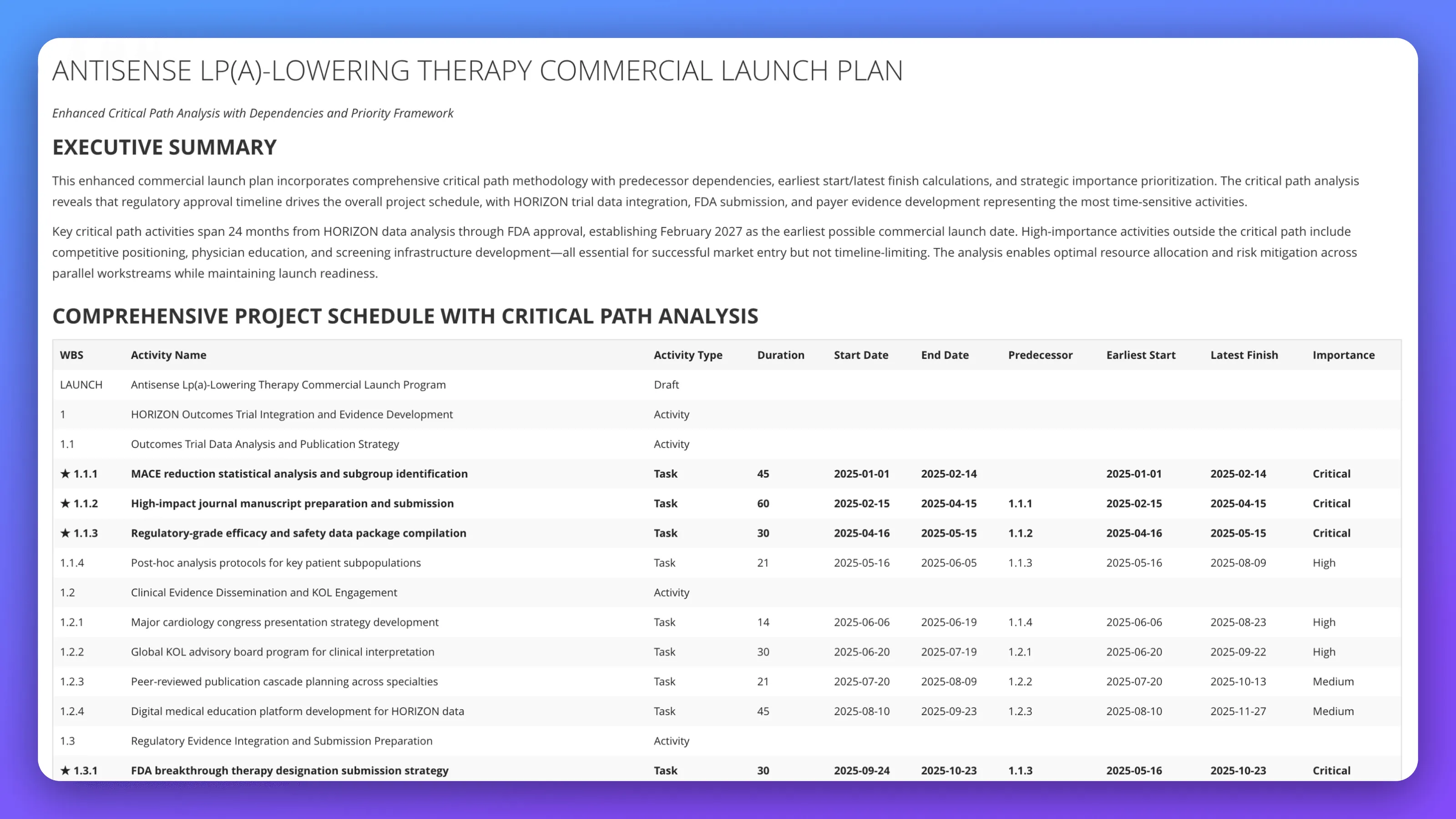
Build commercial launch execution plan for antisense Lp(a)-lowering therapy advancing from HORIZON Phase III data to market entry; define critical path analysis integrating MACE reduction statistical analysis, regulatory-grade efficacy compilation, FDA breakthrough therapy submission strategy, and KOL engagement; design parallel workstreams for competitive positioning, physician education, and payer evidence development; address timeline optimization preserving February 2027 launch date while managing dependencies across outcomes trial integration, regulatory submission, and market access preparation.
Prompt:
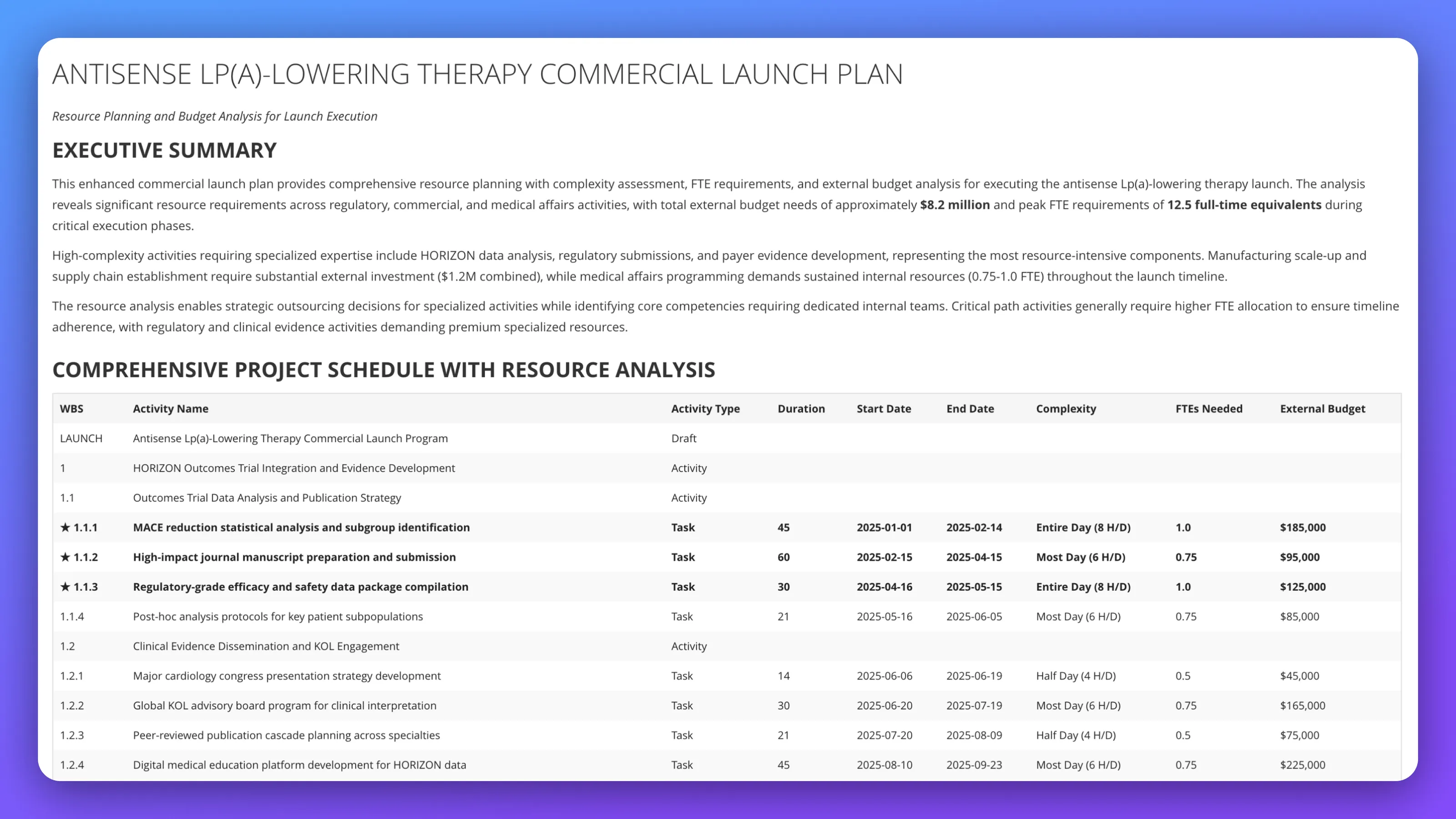
Build resource planning and budget analysis for antisense Lp(a)-lowering therapy commercial launch execution; define complexity assessment across HORIZON data analysis, regulatory submissions, payer evidence development, and medical affairs programming; determine external budget allocation strategy; address strategic outsourcing decisions while preserving critical path timeline and identifying competencies requiring dedicated internal teams with external budget optimization.
Prompt:
Build comprehensive organizational structure and talent requirements for antisense Lp(a)-lowering therapy commercial launch execution; define distinct roles across functions; design cross-functional collaboration framework integrating capabilities; address expertise requirements for antisense oligonucleotide development, cardiovascular outcomes research, and lipid management with specific skill mapping supporting HORIZON data integration and FDA submission strategy.

Describe any strategy shift, risk, or opportunity. Planner transforms your plan accordingly.
Prompt:
Map strategic scenarios for antisense Lp(a)-lowering therapy commercial launch addressing potential HORIZON trial underperformance: Restricted High-Risk Coverage Gateway with specialty pharmacy networks and step therapy requirements, Delayed Broad Access with extended real-world evidence generation, Premium Pricing Strategy with value-based agreements; quantify each scenario for market penetration timeline, payer coverage restrictions, specialty pharmacy requirements, and revenue impact considering PCSK9 inhibitor precedent and Lp(a) treatment landscape dynamics.

Use Cases
Digital-First Obesity Management Benefit Platform
Managed Healthcare
Builder
PCSK9 Base Editing PreClinical Therapeutic Development
Biotechnology
Scoper
Scalable Hospital-at-Home for Advanced Acute Care Delivery
Healthcare Services
Scoper
Digital Therapeutic Adoption Risk Mitigation and Positioning
Digital Health
Mapper
First-in-Class Antisense Therapy Launch Targeting Lp(a)
Pharmaceuticals
Builder
CAR-NK Global Manufacturing Strategic Scenarios
Biotechnology
Mapper
Global Serialization and Anti-Counterfeit Platform Execution
Healthcare Distributors
Builder
Global Real-Time IoT + Cold-Chain Monitoring AI Logistics
Healthcare Distributors
Scoper
Centralized Fleet vs Regional 3PL for mRNA Cold-Chain
Healthcare Distributors
Comparer
Spatial Proteomics Platform Commercialization Pathways
Life Sciences Tools
Comparer
High-Throughput mRNA Synthesis and LNP Formulation
Life Sciences Tools
Builder
GLP-1 Obesity Therapy Integration Risk Assessment
Managed Healthcare
Mapper
Hemostatic Biomaterial Market Access Enhancement
Healthcare Supplies
Enricher
Augmented Lp(a) Therapy Launch Plan with Benchmarks
Pharmaceuticals
Enricher
Next-Gen Spatial Multiomics Platform Development Strategy
Life Sciences Tools
Scoper
Enhanced Organ-on-Chip Corporate Development Plan
Life Sciences Tools
Enricher
Robotic Pharmacy Fulfillment Excellence Through Benchmarks
Healthcare Distributors
Enricher
Hub-and-Spoke vs. Decentralized Models for Hospital-at-Home
Healthcare Services
Comparer
HER2×PD-L1×VEGF Trispecific Antibody Development Program
Pharmaceuticals
Scoper
Strategic Scenarios for Antisense Lp(a) Therapy Launch
Pharmaceuticals
Mapper
First-in-Class Antisense Therapy for Lp(a) Reduction
Pharmaceuticals
Scoper
AI-Powered Diabetes Remote Monitoring Platform Deployment
Digital Health
Builder
Specialist-First vs Primary Care-First Launch Strategy Analysis
Pharmaceuticals
Comparer
COVID-Era mRNA Precedents for Cancer Vaccine Submission
Biotechnology
Enricher
Digital Twin Hospital Deployment Strategic Scenarios
Healthcare Facilities
Mapper
Global AI-Enabled Mammography Regulatory Resilience
Medical Devices
Mapper
Next-Gen Bidirectional Neural Interface BMI: Launch-Readiness
Medical Devices
Scoper
Modular Biosecure Hospital Pod: Strategic Development and Global Deployment
Healthcare Facilities
Scoper
Precision Nutrition Clinic Network Expansion with Benchmarks
Healthcare Services
Enricher
Biodegradable Surgical Mesh Supply Chain Scale-Up
Healthcare Supplies
Builder
TAVR System Pivotal Trial Execution Alternatives
Medical Devices
Comparer
AI-Powered Population Health Analytics Platform
Managed Healthcare
Enricher
OR Retrofit vs New Smart OR Construction
Healthcare Facilities
Comparer
Hospital Lifecycle Management: AI Digital Twin Integration
Healthcare Facilities
Builder
Global Commercial Launch Plan for a Gene Therapy for DMD
Biotechnology
Builder
Launch Comparison: Bispecific Antibody vs HER2-Low ADC
Pharmaceuticals
Comparer
Cardiovascular Outcomes Phase III for Oral Factor XIa Inhibitor
Pharmaceuticals
Builder
Biodegradable Single-Use vs. Reusable Antimicrobial Surgicals
Healthcare Supplies
Comparer
Smart Wound Dressing with Biosensors + Paced Drug Release
Healthcare Supplies
Scoper
AI-Driven Real-Time Utilization Management Decision Engine
Managed Healthcare
Scoper
Quantum-Enabled Cryo-EM Commercialization Risk Mitigation
Life Sciences Tools
Mapper
PSMA Radioligand Launch: Evidence-Based Optimization
Pharmaceuticals
Enricher
Biodegradable Drug-Eluting Stent Market Launch
Healthcare Supplies
Mapper
Comparing GLP-1 Outcomes-Based Contracting Models
Managed Healthcare
Comparer
Enhanced AI Pathology Compliance Framework
Digital Health
Enricher
Global Net-Zero Hospital Modernization
Healthcare Facilities
Enricher
Soft-Robotic Exoskeleton Lifecycle Management
Medical Devices
Enricher
Lipid Editing Therapies: Strategic Clinical Trial Comparison
Biotechnology
Comparer
Phase III GLP-1 NASH Adaptive Trial: Strategic Scenarios
Pharmaceuticals
Mapper
Global mRNA Vaccine Distribution Crisis Scenarios
Healthcare Distributors
Mapper
MRI Diagnostic SaMD AI Tool: Regulatory Development
Digital Health
Scoper
Precision Oncology Service Line Scenario Analysis
Healthcare Services
Mapper
Virtual Oncology Care Network Expansion Plan
Healthcare Services
Builder
Regulatory Submission Plan for a Robotic Surgical Platform
Medical Devices
Builder
Digital Therapeutic Hypertension Adoption Models
Digital Health
Comparer
Unipr is built on trust, privacy, and enterprise-grade compliance. We never train our models on your data.






Start Building Today
Log in or create a free account to scope, build, map, compare, and enrich your projects with Planner.